Breast Implant Associated ALCL
Breast Implant Associated ALCL develops in the capsule surrounding the breast implant, but in some cases, it can spread to other parts of the body. BIA-ALCL is not a type of breast cancer but lymphoma commonly observed in patients with textured breast implants.
Breast implant patients can experience surgical or implant-related complications at some point, immediately after surgery or years later. The complications can be common or less common with varying degrees of severity. One of these complications is BIA-ALCL.
BIA-ALCL treatment may involve implant removal (total capsulectomy) and other treatment options like replacement surgery and chemotherapy. Despite the low risk of contracting BIA-ALCL, patients considering breast implant surgery should learn about the complications and take the necessary precautions to prevent its occurrence.
What is BIA-ALCL: Is it Breast Cancer?
Breast Implant-Associated Large Cell Lymphoma is a T-cell lymphoma (a type of non-Hodgkin’s lymphoma). The complication is an immune system cancer found in the scar tissue and fluid surrounding the breast implant. Despite its occurrence due to breast implants, BIA-ALCL is not breast cancer, a cancer that forms from breast cells, but a cancer of the lymphatic system.
BIA-ALCL is entirely T-cell and has no anaplastic lymphoma kinase gene translocation. Some breast Implant-Associated ALCL characteristics include abnormal lymphocyte growth with CD30 expression within the peri-implant capsule. It can occur as an effusion between the breast implants and the surrounding fluid and scar tissue or with a lump in the breast or the armpit.
Is BIA-ALCL Common?
BIA-ALCL is uncommon and largely underreported, making it hard to estimate its risk rate due to insufficient information. From the data collected by the Australian Breast Device Registry (ABDR) in 2018, of 5,886 patients who had revision surgery, the rate of BIA-ALCL was 4 of every 1000 patients. The overall estimated risk of BIA-ALCL is between 1-in-2,000 and 1-in-36,000.
All Australian cases of BIA-ALCL have involved textured implants or implants with polyurethane coating. There are no cases involving smooth implants. The complication’s symptoms mainly appear between three to fourteen years after the procedure, with the average occurrence time estimated to be seven to eight years.
When was the First Case of BIA-ALCL Discovered?
The first case of BIA-ALCL was reported in 1997 in a 41-year-old woman who developed a CD30+ peripheral T-cell lymphoma mass. The mass was found in the fibrous capsule surrounding her cosmetic textured-surface breast implant. Since 1997, more than 500 unique cases have been reported in 23 countries worldwide.
How Many BIA-ALCL Cases Are There in Australia?
Initially, the number of BIA-ALCL reported cases was relatively low. However, the number of reported cases has grown over the years. There are over 100 confirmed cases of BIA-ALCL in Australia.
Symptoms of BIA-ALCL

BIA-ALCL symptoms develop at least one year after breast implant surgery and, on average, seven to ten years. The symptoms often lead to breast shape and size changes, with one breast looking different if the complication affects one breast. BIA-ALCL symptoms usually arise at the breast implant site. The surgeon will find fluid collection around the implant (seroma) on evaluation.
The common symptoms of BIA-ALCL include:
- Effusion surrounding the breast implant
- Breast hardening
- Breast enlargement and swelling
- Asymmetry
- Pain around the breast and armpit
- Lumps in your breast or around the armpit
- Skin rash and reddening around the breast (occurs rarely)
However, patients should note that symptoms like swelling occur immediately after the procedure, and it’s completely normal. In addition, breast hardening can result from capsular contracture. Other complications like bacterial infection and implant rupture can exhibit the same symptoms as BIA-ALCL. Also, effusion surrounding the breast implant should not be mistaken for implant rupture.
Therefore, you should contact your surgeon for testing to determine the complication.
BIA-ALCL Aetiology
Like most cancers, the Breast Implant Associated ALCL aetiology is complex and multifactorial, with the complete causing factors unknown. The complication is common in patients with textured breast implants. However, breast implants are unlikely to be the sole cause of BIA-ALCL. Some factors proposed by medical researchers that are likely to cause BIA-ALCL include:
Bacterial Infection, Causing Inflammation
The bacteria multiply on the implant’s surface, forming a resistant biofilm. This results in chronic inflammation. Chronic inflammation produces chronic antigens, causing the immune cells to become malignant. The cells mutate, becoming cancerous.
Genetic Predisposition

Patients respond differently to infections depending on their immune system. Some studies have highlighted the germline TP53 BRCA 1/2 gene mutations as a possible risk factor for developing BIA-ALCL. This is based on the reported higher incidence of BIA-ALCL in patients with Li-Fraumeni Syndrome and BRCA 1/2 carriers.
However, research has not identified any difference between bacteria found in BIA-ALCL patients and those without complications. This shows that BIA-ALCL may rely on an individual’s genetics and immune response. More studies need to be carried out regarding this factor.
Time Before the Process Develops
Some theories suggest silicone contributes to inflammation (silicone autoimmunity), while others suggest that viruses may also be a contributing cause. However, there’s no risk difference between silicone and saline breast implants. It is also important to note that BIA-ALCL risk is not affected by implant rupture or reconstruction surgery, whether primary or after the cancer.
Implant Texture
The risk of BIA-ALCL increases in highly textured breast implants. Medical experts suggest that textured implants offer a large surface area for bacterial growth. The bacteria may result from contact between the breast implant and the external tissues during insertion. The bacteria contributing to BIA-ALCL are commonly found on the skin.
Textured Breast Implants and BIA-ALCL
Researchers note that textured breast implants increase the risk of BIA-ALCL; the greater the texturing, the higher the risk. In Australia, most reported instances of BIA-ALCL involve patients with textured breast implants or who had textured breast implants at one point.
Surgeons adopted the use of textured breast implants to reduce the rate of implant rotation and malposition. However, medical experts note there’s no association of the disease with the type of material used in breast implants (saline or silicone) or the shape (teardrop or round).
Textured breast implants associated with BIA-ALCL are Polyurethane Silimed and Allergen Biocell. Reported BIA-ALCL cases to TGA (Therapeutic Goods Administration) include:
- Polyurethane-coated breast implants, with an approximated risk of 1 in 1800
- Macro-textured breast implants, with an approximated risk of 1 in 2,400
- Micro-textured breast implants, with a compared risk of 1 in 18,000
There’s no evidence showing an association between smooth breast implants and BIA-ALCL. TGA reports no confirmed BIA-ALCL cases in patients with a history of only smooth breast implants (except for 2 potential cases from FDA).
TGA has banned the usage of macro-textured breast implants, including Polyurethane Silimed and Allergen Biocell, in Australia.
Are Textured Tissue Expanders Associated With BIA-ALCL?
Tissue expanders are primarily used after mastectomy to help stretch the breast tissue and skin before breast reconstruction using a breast implant. They are also used during scar revision, in correcting underdeveloped breasts, and in tissue defect procedures.
Tissue expander usage is only six months. Therefore, there’s limited information to link tissue expanders to BIA-ALCL because the patient is only temporarily exposed to the device. However, note that TGA has also banned Biocell textured tissue expanders.
What to Do if You Have Textured Breast Implants: Should You Remove Them?
If you have the banned textured breast implants and experience symptoms of BIA-ALCL and the tests confirm it, the next step is implant removal. However, if you have the implant and experience no symptoms, you don’t have to remove the implant. The Australian TGA doesn’t recommend the removal of textured breast implants as it classifies the risk of cancer as rare.
In addition, surgery for breast implant removal is technically complicated and can cause more harm than good if there’s no clinical reason for removal. Any surgical procedure and anaesthetic have risks. BIA-ALCL has a high rate of cure upon diagnosis.
It’s recommended that the patient should be aware of symptoms of BIA-ALCL and keep a record of the implant’s manufacturer, device identifier, and model name while monitoring any breast changes. Also, the patient should report any symptoms of BIA-ALCL to a qualified specialist surgeon immediately.
What Implant Type Should I Get?
Regarding the suitable breast implant, Dr. Beldholm notes, “In the current climate, I prefer using a smooth implant as there is a minimal risk (as far as we know ) of ALCL. Microtextured/ nanotextured don’t have any benefits.”
Diagnosis of Breast Implant-Associated ALCL
If you have any BIA-ALCL symptoms, the doctor will recommend various diagnostic tests to verify the complication. These tests include:
Clinical Evaluation
The main symptoms of BIA-ALCL are swelling, pain, or lumps in the patient’s breast. These symptoms may indicate the presence of fluid collection around the implant or a mass in the capsule. A physical examination and medical history are performed to assess the type and duration of the implants and any previous complications.
Ultrasound
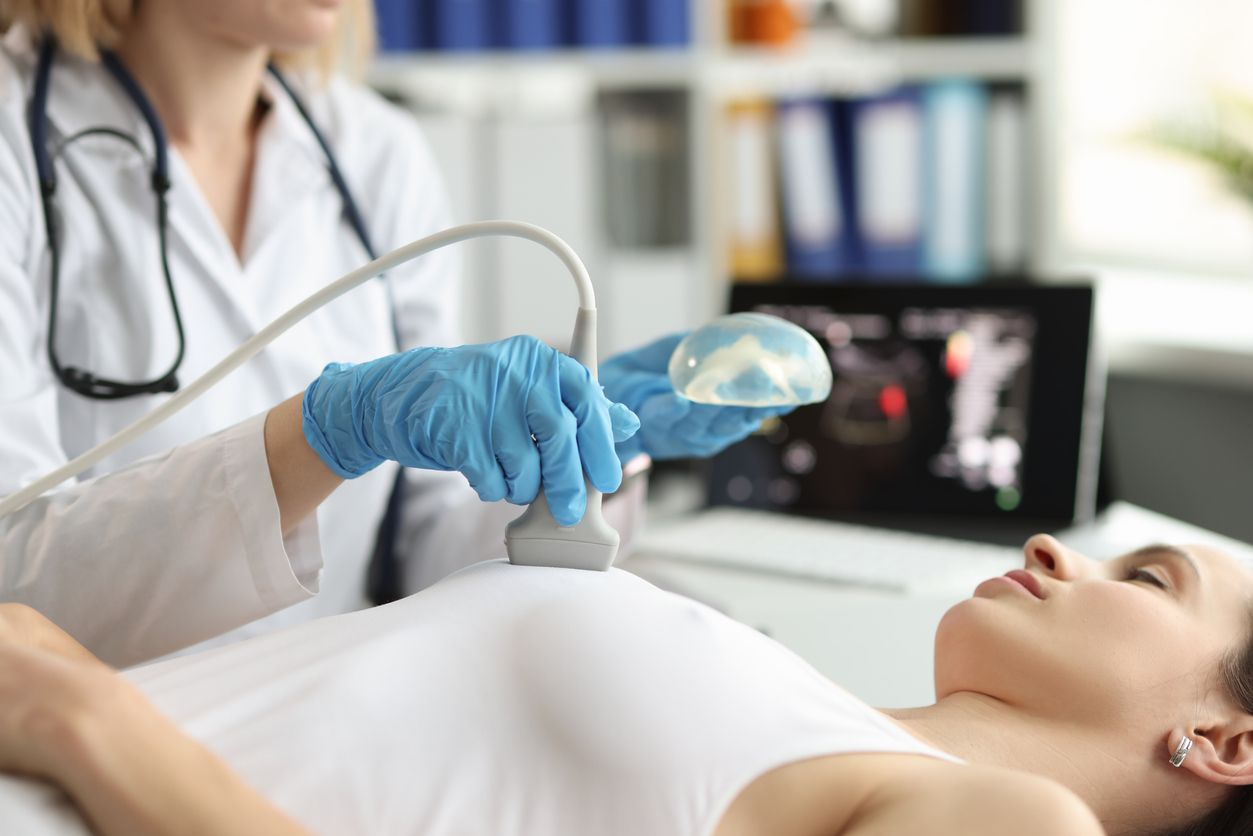
The Imaging test is the first test the surgeon will recommend to determine if there are lumps or fluid accumulation in the patient’s breast. The test can also show any lumps in lymph nodes. MRI may be a complementary test to provide more detailed information about implant integrity, capsule thickness, and tumour invasion.
Laboratory Testing
The tissue or fluid from the biopsy is subjected to microscopic examination and biomarker testing to identify cancer cells. The definitive diagnosis of BIA-ALCL requires cytologic or histologic confirmation of abnormal CD30-positive T-cells in the fluid or tissue sample from the affected breast.
A specialist surgeon can obtain the fluid by fine needle aspiration or drainage of the implant. On the other hand, they can get the tissue by biopsy or capsulectomy. The samples are later processed to produce smears, cell blocks, and histologic sections for staining and immunohistochemistry. Molecular testing, such as polymerase chain reaction (PCR) for T-cell receptor gene rearrangement, can be performed to detect clonality.
If BIA-ALCL is confirmed, the surgeon may recommend an MRI (Magnetic Resonance Imaging) or a CT (Computed Tomography) scan to determine the spread of the disease. PET (Positron Emission Tomography) scan also determines the spread. Note that despite mammogram tests usage for the cancer detection, they don’t detect BIA-ALCL.
Treatment of BIA-ALCL

Treatment of BIA-ALCL involves the combined effort of a multidisciplinary team, including a breast implant specialist surgeon, a blood disease specialist experienced in lymphoma, and a cancer specialist. The team will then develop a treatment plan.
The primary treatment option for BIA-ALCL is implant removal and the surrounding capsule (total capsulectomy). Studies show that surgical management for BIA-ALCL is sufficient in the early-stage. This applies primarily to cases with cancer only within the fluid surrounding the breast implant. The surgery involves the removal of all implants irrespective of whether the complication has occurred on one breast.
Where the patient had subpectoral implant placement, adherence to the rib cage can make complete resection difficult, while an injection of tumescent solution facilitates complete excision. In this case, care must be taken to avoid pneumothorax
In cases where the cancer spreads to the surrounding capsule, the surgeon will recommend further treatment like additional surgery, radiotherapy, or chemotherapy.
Reducing the Risk of BIA-ALCL
Below are some of TGA (Therapeutic Goods Administration) recommendations on BIA-ALCL and steps to reduce its risks.
The 14-Point Plan

Textured breast implants and bacterial contamination are the most accepted, though not fully proven, causes of BIA-ALCL. Medical researchers recommend that plastic surgeons follow a fourteen-point plan to reduce contamination during breast surgery, reducing the risk of BIA-ALCL. The method includes:
- Using intravenous antibiotic prophylaxis at the time of anaesthetic induction
- When possible, using inframammary incisions
- Using nipple shields to cover the nipples and prevent spillage of bacteria onto the skin
- Performing careful atraumatic dissection to minimise devascularised tissue
- Performing careful prospective hemostasis
- Avoiding dissection into the breast parenchyma
- Using submuscular and/or dual-plane pocket
- Irrigation of the entire breast implant pocket
- Steps to minimize skin-to-implant contact include the use of a sleeve, adequate incision size, skin barrier, Keller funnels, and re-prepping skin with an antibiotic solution or skin prep
- Minimising the time of implant opening, repositioning, and replacement
- Changing surgical gloves before handling the implant and cleaning all instruments with an antibiotic solution or using new instruments
- Avoiding the use of a drainage tube for primary augmentation; in revision and reconstruction cases, use proper technique when a drain is necessary
- Using a layered closure
- Recommending that your patients who have undergone breast surgery take antibiotic prophylaxis before procedures that breach skin or mucosa (i.e. tattoos, piercings, and dental procedures)
Ongoing Clinical Surveillance
Early detection of BIA-ALCL helps in treatment, preventing the spread of the disease. Therefore, regular breast monitoring and screening will help reduce the risk of BIA-ALCL.
Breast implant patients are recommended to regularly self-examine their breasts and visit their GP or surgeon every 12 months for a clinical review to assess any complications and other concerns.
In addition, patients should be:
- Advised to become familiar with the usual features of their breasts
- Made aware of the common symptoms of BIA-ALCL and other implant-related complications
- Conduct regular self-examinations as they would for routine breast cancer awareness
- Encouraged to present for immediate clinical assessment should there be any change in size, shape, or symptoms related to the breast and/or implant.
Reporting BIA-ALCL Cases
Patients and surgeons are encouraged to report any problems arising from breast implants to help investigate these complications. The reporting channels include the TGA reporting channel or ABDR (Australian Breast Device Register). Stay in the know, and stay safe.
Bottom Line
Breast Implant-Associated Anaplastic Large Cell Lymphoma is a rare cancer associated with breast implants. The complication is common in patients with a history of textured breast implants.
Despite the low risk of contracting, the complication can be life-threatening if left untreated. To reduce the risk of BIA-ALCL, patients should monitor their breast implants regularly to identify any related symptoms. On the other hand, surgeons should avoid using textured breast implants and employ techniques that reduce bacterial contamination during implant insertion.
References
- Adams, W. P., Culbertson, E. J., Deva, A. K., Magnusson, M., Layt, C., Jewell, M. L., Mallucci, P., & Hedén, P. (2017). Macrotextured Breast Implants with Defined Steps to Minimize Bacterial Contamination around the Device: Experience in 42,000 Implants. Plastic and Reconstructive Surgery, 140(3), 427–431.
- Longo, B., Di Napoli, A., Curigliano, G., Veronesi, P., Pileri, S., Martelli, M., De Vita, R., Felici, N., Cirillo, P., Bernardi, C., D’Orsi, G., Giacalone, M., Storti, G., & Cervelli, V. (2022). Clinical recommendations for diagnosis and treatment according to current updated knowledge on BIA-ALCL. The Breast, 66, 332–341.
- Kolasinski, J., Sorotos, M., Firmani, G., Panagiotakos, D. B., Płonka, J., Kolenda, M., & Di Pompeo, F. S. (2023). BIA-ALCL epidemiology in an aesthetic breast surgery cohort of 1501 patients. Aesthetic Surgery Journal.
- O’Connell, R., Sharma, B., El-Sharkawi, D., Wotherspoon, A., Attygalle, A., MacNeill, F., Khan, A., & Tasoulis, M. (2023). ASO Visual Abstract: Oncological Outcomes after Multidisciplinary Management of Breast Implant-associated Anaplastic Large Cell Lymphoma (BIA-ALCL). Annals of Surgical Oncology.
- Administration, T. G. (2023c, July 12). Breast implant associated cancer (BIA-ALCL): Information for consumers. Therapeutic Goods Administration (TGA).
- Keith, L., Herlihy, W., Holmes, H., & Pin, P. G. (2017). Breast Implant-Associated Anaplastic large cell lymphoma. Baylor University Medical Center Proceedings.
- Deva, A. K., Turner, S. D., Kadin, M. E., Magnusson, M., Prince, H. M., Miranda, R. N., Inghirami, G., & Adams, W. P. (2020a). Etiology of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL): Current directions in research. Cancers, 12(12), 3861.
- Di Pompeo, F. S., Sorotos, M., Clemens, M. W., Firmani, G., Athanasopoulos, E., Arctander, K., Berenguer, B., Božikov, K., Cardoso, A. C. C., Nord, A., Filip, C., Romania, A., Heitman, C. K., Kaarela, O., Kolenda, M., Hamdi, M., Lantieri, L., Lumenta, D. B., Mercer, N., Vranckx, J. (2020). Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL): Review of epidemiology and prevalence assessment in Europe. Aesthetic Surgery Journal, 41(9), 1014–1025.
- Nelson, J. A., McCarthy, C. M., Dabic, S., Polanco, T. O., Chilov, M., Mehrara, B. J., & Disa, J. J. (2021a). BIA-ALCL and Textured Breast Implants: A Systematic Review of Evidence Supporting Surgical Risk Management Strategies. Plastic and Reconstructive Surgery, 147(5S), 7S-13S.
- Lee, J. (2021). Breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL). Yeungnam University Journal of Medicine, 38(3), 175–182.
- De Faria Castro Fleury, E. (2020). Breast Implant-Associated Anaplastic large cell lymphoma (BIA-ALCL): an open wound. Aesthetic Plastic Surgery, 44(2), 627–629.
- Bachour, Y. (2021b). Capsular contracture in breast implant surgery: Where are we now and where are we going? Aesthetic Plastic Surgery.
- Administration, T. G. (2023b, February 7). BIA-ALCL: Information for health professionals. Therapeutic Goods Administration (TGA).
- Sharma, B., Jurgensen-Rauch, A., Pace, E. M., Attygalle, A. D., Sharma, R. K., Bommier, C., Wotherspoon, A., Sharma, S., Iyengar, S., & El-Sharkawi, D. (2020). Breast implant–associated Anaplastic large cell lymphoma: Review and Multiparametric Imaging Paradigms. Radiographics, 40(3), 609–628.