Do I Need to Change My Breast Implants Every 10 Years?
Breast implants are not lifetime devices and may require replacement at some point. However, you don’t have to replace breast implants after every ten years because there’s no predetermined lifespan of breast implants.
Despite numerous improvements, most women undergoing breast augmentation are concerned about the lifespan of their breast implants. Different sources offer different answers, with most suggesting that breast implants have a ten-year lifespan, requiring replacement after the period.
However, this is not the case, and many reasons lead to implant replacement. These include implant-related complications like capsular contracture, implant rupture, BIA-ALCL, and individual factors like breast size and shape change.
Breast Augmentation
What’s the Breast Implant’s Lifespan?
Breast implants don’t last forever. However, as many believe, they don’t have a prespecified lifespan of ten years. Modern implants can last longer, with some women having them in place for over twenty years. This is because a breast implant’s lifespan is affected by various factors, including the occurrence of complications like implant rupture.
You only need your breast implants replaced if you have complications; if they function perfectly, you don’t need a replacement. However, the older the implant, the higher the risk of complications. On average, the risk of implant rupture increases by one percent every year. Also, individual patient factors determine the implant’s lifespan; hence, the timeline differs in different patients.
Taking care of the breast implants and undergoing regular monitoring will help prolong their lifespan and reduce the likelihood of replacing breast implants. According to the Australian Breast Device Registry (ABDR) report in 2021, the all-cause revision for cosmetic procedures at six years was 5.6%, and revision due to complications was 3%. A report by the FDA in 2011 showed that only 20% of women undergo implant replacement in ten years; this means that 80% of women have their breast implants intact for more than ten years.
In addition, newer-generation silicone breast implants have a stronger shell and thick silicone gel, reducing implant rupture risk and, hence, breast implants last longer.
Factors Leading to Breast Implant Removal/ Replacement
Breast implants may require removal, with or without replacement, at some point. Some of the reasons for revision include implant-related complications or a personal decision by the patient. Below are the common factors leading to the removal of breast implants.
Capsular Contracture
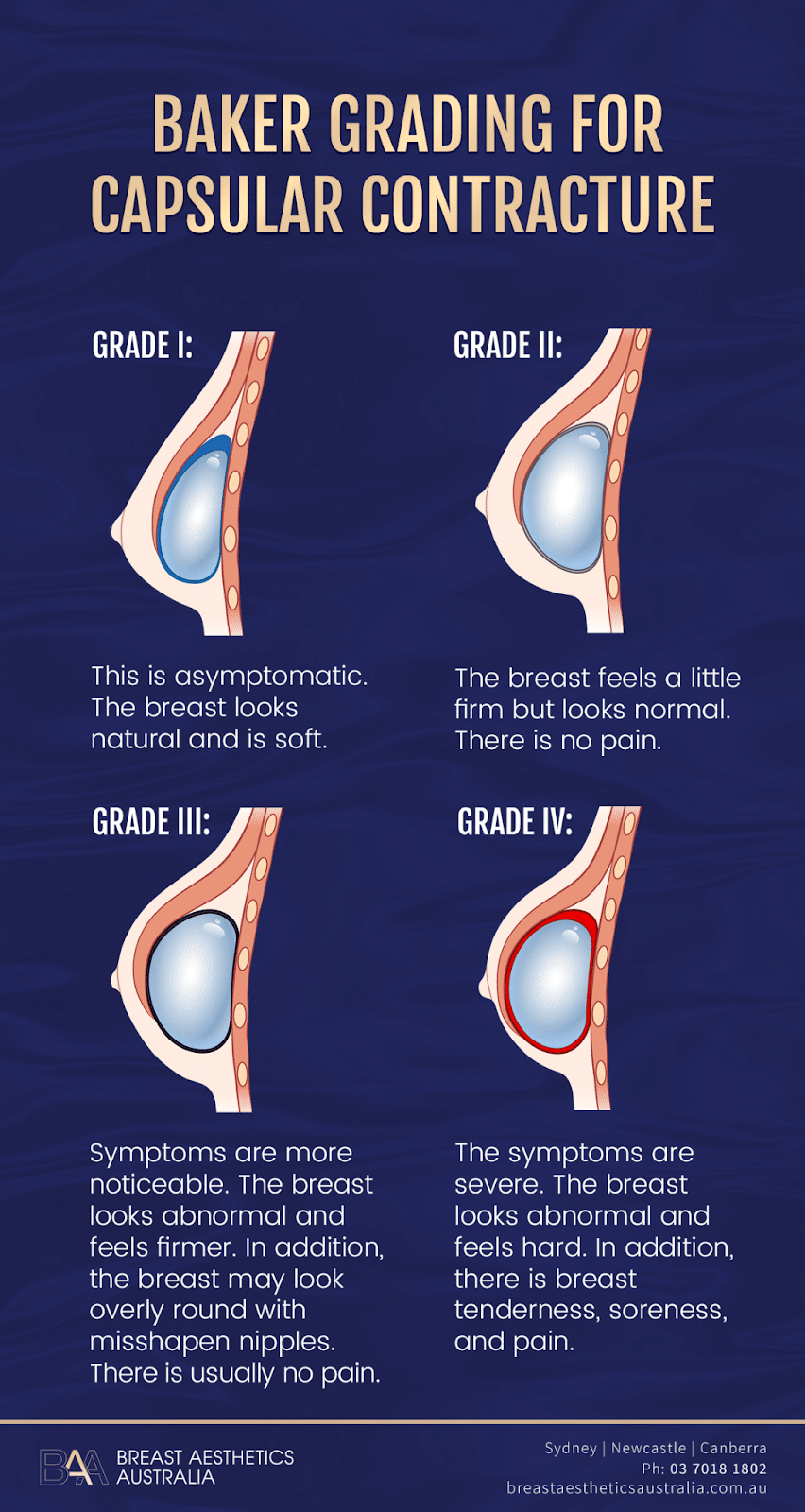
Baker grading for capsular contracture | BAA
Capsular contracture is a common breast implant complication and a leading cause of revision surgery. It occurs when the scar tissue surrounding the breast implant becomes hard and tight, causing the breasts to feel abnormally hard, tender, and painful in some instances. Capsular contracture can occur immediately or years after surgery in one or both breasts.
Capsular contracture has an occurrence rate of 13.5% and higher. In addition, it’s progressive, and the longer the period under consideration, the higher the cumulative rate of occurrence.
However, despite being a common breast implant-related complication, there’s no agreement on the aetiology of capsular contracture. Leading theories suggest the alignment and contraction of fibroblasts within the capsule. The fibroblasts result from the body’s immunological response to the introduction of a foreign body (breast implant). This theory notes that the higher rates of capsular contracture in patients with smooth breast implants result from the fibroblasts forming a planar arrangement on the implant’s surface, allowing contraction.
Other theories explaining the occurrence of capsular contracture include inflammatory reactions resulting from bacteria contamination and genetic predisposition. In addition, radiation during chemotherapy, periareolar incision, sub-glandular placement, and seroma and haematoma significantly increase the risk of capsular contracture.
How’s Capsular Contracture Treated?
Capsular contracture occurs in four levels: grade one, two, three, and four. The standard treatment options for capsular contracture are capsulectomy and capsulotomy. Sometimes, the surgeon may suggest ultrasound therapy or other non-surgical options like injectables and closed capsulotomy.
One way to reduce the risk of capsular contracture includes using textured breast implants. The irregular surface of textured breast implants disrupts the planar arrangement of fibroblasts. However, textured breast implants, especially macro textured implants, pose a risk of BIA-ALCL.
Other ways to reduce the rate of capsular contracture include submuscular placement, inframammary incisions, and the no-touch breast insertion technique. Capsular contracture may reoccur after treatment.
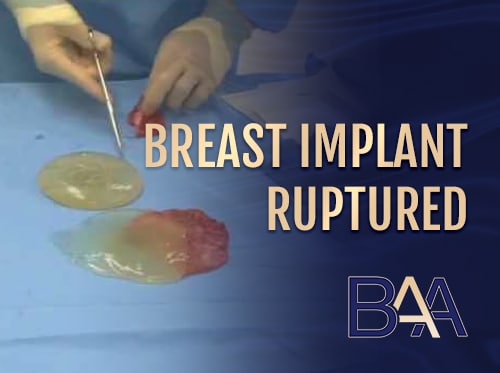
Implant Rupture
Ruptured breast implant | BAA
Rupture occurs when a breast implant breaks or tears, whether it causes size changes or deflation. Saline breast implant ruptures are highly noticeable because the implant deflates, leading to breast size and volume loss. On the other hand, silicone implant ruptures are hard to detect and are referred to as silent ruptures because there are no noticeable signs.
When saline implants rupture, the saline leaks and is absorbed into the surrounding tissues. The saline solution is sterile and harmless. However, there are issues with silicone implant ruptures causing autoimmune disease. This is an area of concern, with different studies providing contradicting opinions, some claiming a relationship and others claiming no relationship between silicone and the disease.
Saline implant ruptures are detectable through clinical examination, but you’ll require mammography or MRI (Magnetic Resonance Imaging) to detect silicone rupture. However, mammograms are less effective than MRIs in detecting silicone implant rupture. In addition, pressure from a mammogram may rupture an implant or cause the silicone gel from a ruptured silicone implant to leak outside the capsule.
Implant rupture has an occurrence rate of 8.7%, and treatment involves implant removal with or without replacement. However, this can be challenging in silicone implants where the silicone has leaked outside the capsule. Breast augmentation patients should undergo MRI screening three years after surgery and every year after that. Note that the risk of implant rupture and capsular contracture increases after revision surgery.
Breast Implant Associated-Anaplastic Large Cell Lymphoma (BIA-ALCL)
“Breast Implant Associated-Anaplastic Large Cell Lymphoma (BIA-ALCL) is a rare immune system cancer associated with breast implants. BIA-ALCL is not breast cancer (cancer developing from cells in the breast).” It grows in the hardened scar tissue and fluid surrounding the breast implant.
BIA-ALCL can occur immediately after surgery or years later, with an average time to diagnosis of eight years and a 0.003% occurrence rate. The common signs and symptoms of BIA-ALCL include swelling, lumps, rashes, and pain around the armpits and breast area resulting from fluid build-up around the scar tissue.
Macro textured breast implants are associated with an increased risk of BIA-ALCL. One occurrence theory suggests that “bacterial contamination introduced at the time of breast implant surgery may over time lead to a biofilm that triggers an inflammatory and immune system response. This response, in conjunction with genetic predisposition, may over time lead to BIA-ALCL.” Textured breast implants have a greater surface area, allowing for greater amounts of bacteria.
Another study notes that “the immune system might also respond to a component of textured surfaces, and a rough surface could be irritating or abrasive, increasing the risk of an inflammatory response.”
How’s BIA-ALCL Detected and Treated?
At the Breast Aesthetics Australia Clinic, the surgeon will examine a patient who develops a palpable mass or fluid collection around the implant. The evaluation involves:
- Ultrasound scan to detect fluid
- If the test detects any fluid, it is removed and sent for laboratory analysis to confirm BIA-ALCL
- If BIA-ALCL is confirmed, the plastic surgeon may order breast lift, a CT scan or an MRI to detect the spread of cancer. PET scans can also be performed to detect the spread.
Mammographs are not effective in detecting BIA-ALCL.
Treatment of BIA-ALCL involves removing the breast implant and the surrounding capsule. The surgeon removes one or both implants even if the cancer affects only one implant. In addition, the surgeon can recommend radiotherapy or chemotherapy if the cancer has spread.
TGA, ABDR, and other medical craft groups in Australia don’t recommend the removal of textured breast implants in asymptomatic women. However, TGA has banned the usage of macro-textured breast implants in Australia.
Malposition
Implant malposition occurs when the breast implant moves from the original position in the breast pocket. This can result from age, gravity, or significant weight gain, among other factors. Your surgeon will recommend breast reconstruction to correct malposition.
Implant Visibility and Rippling
There are cases where the implant edges become noticeable on the skin, especially in women with inadequate breast tissue. In addition, you can experience implant wrinkling or rippling, which is common in saline implants. Implant revision surgery corrects these complications.
Individual Choice
Some patients may be dissatisfied with the results of the surgery and consider implant removal. In addition, some patients may consider breast implant replacement to change the implant size or shape. This is why you should consider breast augmentation consultation before your surgery.
What if I Don’t Replace My Breast Implants?
You don’t have to replace your breast implants unless they have complications. However, most breast implant complications result from implant aging, not surgery. Therefore, failure to replace them may increase the risk of complications.
Bottom Line
Breast implants will require replacement during your lifetime since they don’t last forever. However, you don’t have to replace breast implants after every ten years. The common reason for implant removal or replacement is complications, including implant rupture, capsular contracture, and BIA-ALCL. Taking care of the implants will help to prolong their lifespan. Note that most women have had implants for over twenty years without replacement. Therefore, as stated by many, the ten-year “fact” is just but a myth.
References
- Nelson, J. A., McCarthy, C. M., Dabic, S., Polanco, T. O., Chilov, M., Mehrara, B. J., & Disa, J. J. (2021). BIA-ALCL and Textured Breast Implants: A Systematic Review of evidence supporting Surgical Risk Management Strategies. Plastic and Reconstructive Surgery, 147(5S), 7S-13S.
- Administration, T. G. (2023, September 15). Breast implant associated cancer: consumer information. Therapeutic Goods Administration (TGA).
- Handel, N., Cordray, T., Gutierrez, J., & Ja, J. (2006). A Long-Term Study of Outcomes, Complications, and Patient Satisfaction with Breast Implants. Plastic and Reconstructive Surgery, 117(3), 757–767.
- Groth, A. K., & Graf, R. (2019). Breast Implant-Associated Anaplastic Large cell lymphoma (BIA-ALCL) and the textured breast implant crisis. Aesthetic Plastic Surgery, 44(1), 1–12.
- Swezey, E. (2023, January 16). Breast implant rupture. StatPearls – NCBI Bookshelf.
- Gabriel, S. E., Woods, J. E., O‘Fallon, W. M., Beard, C. M., Kurland, L. T., & Melton, L. J. (1997). Complications Leading to Surgery after Breast Implantation. The New England Journal of Medicine, 336(10), 677–682.
- Administration, T. G. (2023a, July 12). Current status of breast implant products in Australia. Therapeutic Goods Administration (TGA).
- Zuckerman, D. M. (2010). Reasonably safe? Breast implants and informed consent. Reproductive Health Matters, 18(35), 94–102.
- Calobrace, M. B., Schwartz, M. R., Zeidler, K. R., Pittman, T. A., Cohen, R., & Stevens, W. G. (2017). Long-Term safety of textured and smooth breast implants. Aesthetic Surgery Journal, 38(1), 38–48.